
- Foundation
-
Projects

Documentary ARI, A story of love and life
The documentary ARI, A story of love and life, funded by the Glòria Soler Foundation, is a tribute to Ariana Benedé, her family and all the medical professionals and people and institutions that made it possible to promote the clinical trials that have led to the incorporation of CAR-T therapies, with the aim of curing acute lymphoblastic leukaemia in as many cases as possible.
Read more

MORTALES+ Community, Art and HIV/AIDS
Fundació Glòria Soler, an organisation that has supported research into HIV/AIDS, is supporting a community arts and research project related to the disease and its cures. The project seeks to create spaces for collective reflection through the activation of working groups, which function as mutual support groups.
Read more

Blue Soler Therapeutic SUP. Biomedical research project
The Glòria Soler Foundation supports the Blue Soler Therapeutic Project which aims to generate knowledge to be able to demonstrate the benefits of Stand Up Paddle in the health of adults with musculoskeletal diseases.
Read more
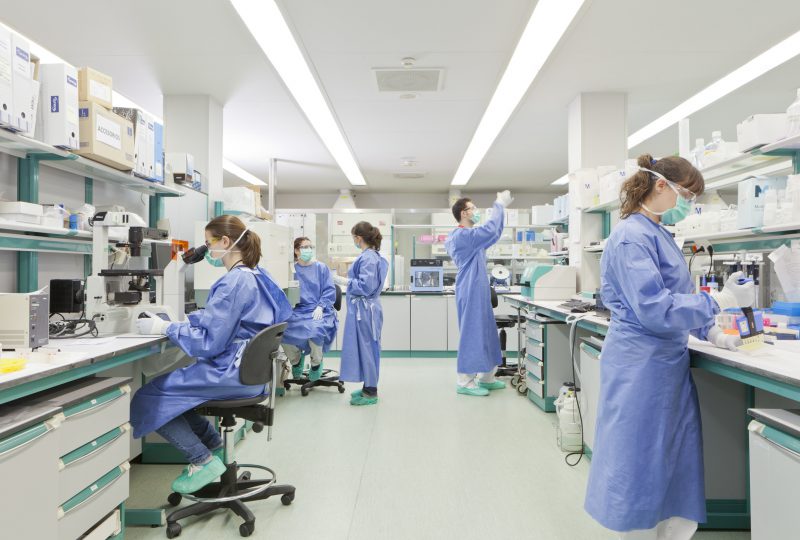
HIV/AIDS vaccine
In collaboration with IrsiCaixa, the Institut de Recerca de la Sida led by Dr. Bonaventura Clotet, the Fundació Glòria Soler has contributed to the advancement of HIV/AIDS research by supporting a therapeutic vaccine to treat HIV/AIDS and reduce the associated adverse effects.
Read more

Suñol Soler Collection
The Glòria Soler Foundation collaborates strategically with the Fundació Suñol, an entity dedicated to the dissemination and promotion of art that has one of the most important collections of contemporary art in the entire State, with a fund of more than 1,000 works by 250 artists. Together with the Glòria Soler Foundation, they work to disseminate contemporary art and bring its values closer to society.
Read more
-
News

The ARI Project at Hospital Clínic-IDIBAPS receives the 2025 National Scientific Patronage Award
The ARI Project at Hospital Clínic-IDIBAPS, supported by the Fundació Glòria Soler, has been recognised with the 2025 National Scientific Patronage Award, presented by the Catalan Foundation for Research and Innovation as part of the National Research Awards, for the collective patronage model that has promoted the development of the first CAR-T therapies integrated into the European public health system.
Read more

Blue Soler Therapeutic SUP has won the award for best research project 2025 from the Catalan Sports Foundation
This first pilot study of the Blue Soler project in 2024 involved an initial group of seven volunteer patients, and was completed this year, 2025, with a second group of eight more patients who completed the programme as a protocolised clinical study. All participants have disabling musculoskeletal diseases of autoimmune origin.
Read more

Blue Soler Project: Stand Up Paddle Surfing as a Therapeutic Tool for Patients with Musculoskeletal Disorders
Following the success of the pilot study carried out in 2024 with very favourable results, the major innovation in 2025 has been that Blue Soler is now being considered as a clinical trial, having obtained approval from the Ethics Committee of the Sant Joan de Déu Hospital.
Read more

Qualitative Research in Health – Social Science & Medicine has published the results of the Covid-19 Africa Study
The July 2025 results of the project funded by the Glòria Soler Foundation have been published in the prestigious journal Social Science & Medicine – Qualitative Research in Health.
Read more

Art and Health at the 7th Conference organised by Torrents d’Art and the Parc Sanitari de Sant Joan de Déu to highlight the impact of culture on emotional wellbeing
Torrrents d’Art and Parc Sanitari de Sant Joan de Déu delve into culture and art as assets for health and happiness. From 5 to 18 May, the programme “Tastets d’art” will also be held, with the participation of the Fundació Suñol and the Fundació Glòria Soler through the educational activity “Mental State” based on the work of the artist Zush from the Suñol Soler Collection.
Read more
- Collection









